PIPELINE
BIOMUNEX is focused on the development of innovative disruptive biological approaches for the treatment of cancer
BIOMUNEX’ pipeline includes a large panel of BiXAb® bispecific antibodies in development and several disruptive biology driven programs for oncology, focused on innovative targets or pairs of targets that should become breakthrough therapies in the future.
Our flagship program uses our BiXAb® antibody platform to redirect a unique, non-conventional T-Cell sub-population (MAIT cells) to specifically engage and kill cancer cells and overcome some of the limitations of classical CD3-based T-cell engagers. This BMX-500 program of BIOMUNEX, started and co-invented in collaboration with the “Cancer and Immunity” Unit at the Institut Curie led by Dr. Sebastian Amigorena, Director of Institut Curie’s Cancer Immunotherapy Center, and Dr. Olivier Lantz, Director of the Clinical Immunology Laboratory at the Institut Curie, who discovered the MAIT cells, focuses on developing novel disruptive and potentially universal T cell redirection approach, the MAIT engagers.
This disruptive MAIT redirection approach aims to overcome some of the limitations of current CD3-based T cell redirecting therapeutic strategies, such as cytokine release storm and subsequent dose-limiting toxicity. This novel strategy may result in a major advancement in the immunotherapy of cancers for both solid tumors and hematological malignancies.
BMX-500 series: MAIT engagers
BiXAb-mediated redirection of MAIT (Mucosal Associated Invariant T) cells
BIOMUNEX has invented (together with Institut Curie) the MAIT engager approach. BIOMUNEX is the first company to be focused on the redirection of MAIT cells for the treatment of cancer by generating MAIT engagers with the BiXAb® technology platform. BIOMUNEX is rapidly advancing this approach towards clinical development in solid tumors.
BiXAb-MAIT engagers offer an elegant biological solution to the current problems facing classical CD3-based T-cell engagers in the clinic: activation of all T-cell subsets leading to overt cytokine release and activation of the regulatory T cells in the tumor microenvironment that increases local immunosuppression.
Mucosal Associated Invariant T-cells (MAITs)
MAIT cells are a subset of non-conventional cytotoxic T-cells that bridge the innate and adaptive immune system. They are abundant in the blood (up to 20% of CD8 population) and are predominantly found in tissues where their primary role is to kill bacterially and virally infected cells and respond to inflammatory signals. Due to their high propensity to migrate into tissue, they are also found in significant numbers in many solid tumors.
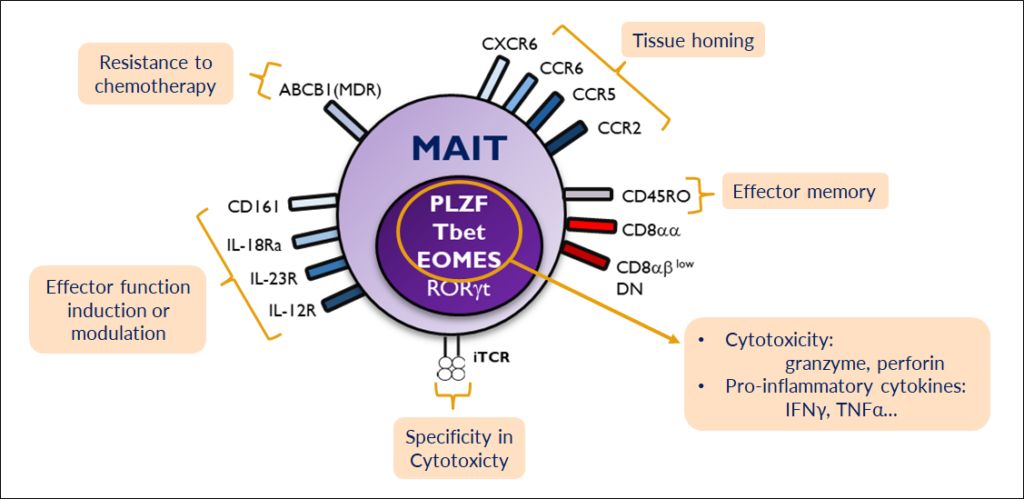
MAIT characteristics
MAIT cells express a semi-invariant T-cell receptor (iTCR) which recognizes bacterial metabolites presented in the context of the MR1 protein (MHC related protein 1) by APCs and infected cells.
They are a potent cytotoxic T-cell subset that can kill in a TCR dependent and independent manner.
MAIT cells express an array of chemokine receptors that mediate tissue infiltration.
They express the multidrug resistance gene and can persist in patients undergoing certain chemotherapies (e.g. anthracyclins).
BiXAb®-MAIT engagers
BIOMUNEX is focused on bringing disruptive innovation to the clinic and is the first company worldwide to be developing MAIT engagers for the treatment of solid tumors. BIOMUNEX strongly believes that MAIT engagers will solve some of the limitations of classic T-cell engagers (TCEs) and will demonstrate efficacy in solid tumors.
Using our best-in-class BiXAb® bispecific antibody platform, we have developed MAIT engagers that are able to uniquely bind the MAIT cells and simultaneously bind a Tumor Associated Antigen (TAA). By bridging the two cells, the MAIT engager effectively creates an immunological synapse that induces MAIT cell activation, degranulation, proliferation and directed cytotoxicity towards the targeted cancer cell.
Advantages of BiXAb-MAIT engagers compared to other T cell engagers:
Due to the MAIT characteristics and superior features of BiXAb antibodies, BiXAb-MAIT engagers have several advantages:
MAIT engagers only activate the MAIT cell population.
MAIT engagers do not activate other T-cell subsets (CD4 and CD8).
Specifically, MAIT engagers do not activate the regulatory T-cell subset (as compared to classical TCEs).
In a PBMC mixture, MAIT engagers, compared to a classical TCE, do not cause a big release of cytokines.
MAIT cells are abundant in the blood, tumors and surrounding tissues.
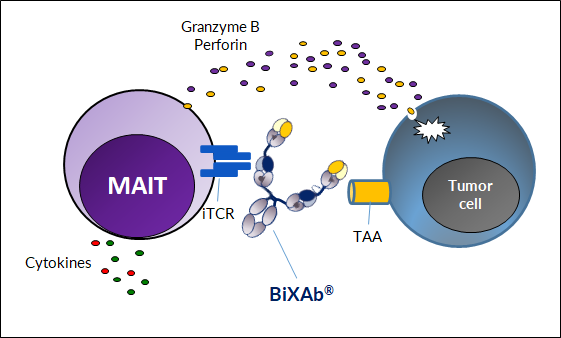
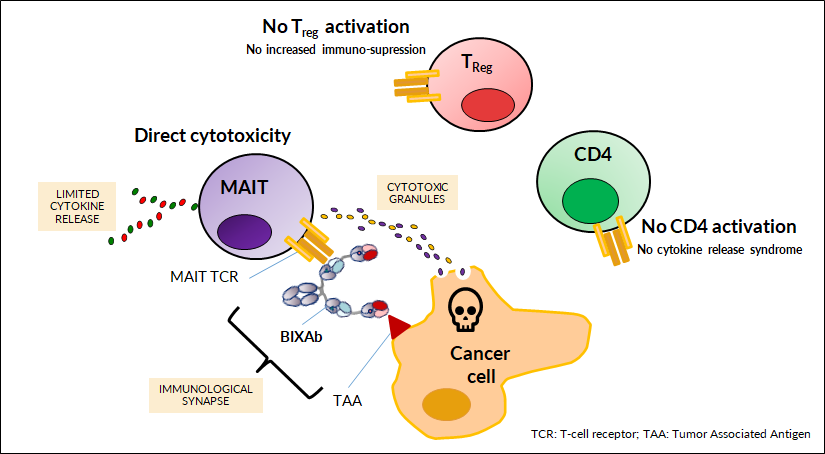
These features of BiXAb-MAIT engagers should lead to reduced cytokine release (a dose limiting toxicity for classical TCEs) which will enable higher dosing (improved solid tumor penetration) and significant efficacy where the Treg population is not activated to increase local immunosuppression. Moreover, BiXAb-MAIT engagers are expected to provide larger therapeutic window compared to classical CD3+ T cell engagers, thanks to the so-called Spark-effect, that enables MAITs to orchestrate immune response.
Other innovative programs:
Other ongoing development candidates target different members of other families of receptors, including an ongoing novel approach of redirection of immune cells.
BMX-003 (HER3 x EGFR BiXAb®)
The BMX-003 drug candidate was selected from screening a series of BiXAbs directed against the ErbB2 family of receptor tyrosine kinases (EGFR, HER2 and HER3) and targets HER3 x EGFR (see article published in Frontiers in Immunology by Rabia et al 2023).
The drug candidate has several demonstrated mechanisms of action (blocking RTK signalling leading to apoptosis, receptor internalization and antibody mediated cellular cytotoxicity induced by NK cells) and this combination provides the potency to impact Kras mutated pancreatic cancers.
The BMX-003 program of Biomunex was developed in collaboration with the team “Immunociblage et Radiobiologie en Oncologie” led by Dr. Pèlegrin at the “Institut de Recherche en Cancérologie de Montpellier” (IRCM). BMX-003 program is based on the co-targeting of two tyrosine kinase receptors (RTKs) on the tumor cell surface, members of the ErbB (HER) sub-family: the Epidermal Growth Factor Receptor (EGFR) and HER3.
BMX-003 BiXAb candidate was designed to bypass the resistance to treatment induced by monotherapy, by simultaneously targeting and blocking the interaction of both EGFR and HER3 with their natural ligands, and by redirecting cytotoxic immune cells against the double targeted tumor cells. It demonstrated strong pre-clinical efficacy in several colon and pancreatic tumor models. BMX-003 BiXAb proved excellent affinity and capacity to co-engage EGFR and HER3, to induce co-degradation of both RTKs, to simultaneously inhibit different downstream intracellular signaling, to induce Natural Killer (NK)-based Antibody Dependent Cell Cytotoxicity (ADCC), but also to reduce neo-angiogenesis, to increase immune cell infiltrate, and to induce tumor growth inhibition in an in vivo pancreatic tumor model.
BMX-003 BiXAb candidate represents an innovative drug to overcome tumor resistance associated with RTK signaling dysregulation, in a wild variety of cancers, notably as a single agent. A nominated development candidate will be developed to clinical Phase 1.